The standard Gibbs energy change for the following voltaic cell is ΔG° = -89.3 kJ at 25 °C.What is E°cell? 
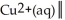
 Ag(s)
Ag(s)
A) +0.463 V
B) -0.926 V
C) -0.463 V
D) +0.926 V
E) +0.231 V
Correct Answer:
Verified
Q59: Will magnesium metal displace Al3+(aq)ion from an
Q60: Determine E°cell at 25 °C for the
Q61: What is E° of the spontaneous cell
Q62: What is the cell reaction of the
Q63: The following galvanic cell has a measured
Q65: For the cell reaction at 25 °C
Q66: Calculate Ecell at 25 °C for the
Q67: Find Ecell at 25 °C for the
Q68: The net reaction in a voltaic cell
Q69: What is Keq at 25 °C of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents