The rate of disappearance of HBr in the gas phase reaction
2 HBr(g) → 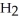 (g) +
(g) + 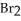 (g) Is 0.130 mol L-1
(g) Is 0.130 mol L-1 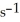
At 150 °C.The rate of reaction is ________ mol L-1 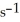
.
A) 3.85
B) 0.0650
C) 0.0169
D) 0.260
E) 0.0860
Correct Answer:
Verified
Q80: In the first-order,reaction A → products,[A] =
Q81: The rate of disappearance of HBr in
Q82: For the second-order reaction A → products,the
Q83: The decomposition of dinitrogen pentoxide is described
Q84: For a reaction with a second order
Q86: For the reaction: A → Products concentration-time
Q87: The decomposition of dinitrogen pentoxide is described
Q88: For a reaction that follows the general
Q89: For the second-order reaction A → products,the
Q90: In the first-order,reaction A → products,[A] =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents