The rate of disappearance of HBr in the gas phase reaction
2 HBr(g) → 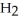 (g) +
(g) + 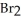 (g)
(g)
Is 0.301 mol L-1 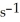 At 150 °C.The rate of appearance of
At 150 °C.The rate of appearance of 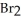 Is ________ mol L-1
Is ________ mol L-1 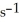
.
A) 1.66
B) 0.151
C) 0.0906
D) 0.602
E) 0.549
Correct Answer:
Verified
Q76: The rate constant at 160 °C for
Q77: Substance A decomposes by a first-order reaction.Starting
Q78: The correct units of the specific rate
Q79: If a catalyst is added to a
Q80: In the first-order,reaction A → products,[A] =
Q82: For the second-order reaction A → products,the
Q83: The decomposition of dinitrogen pentoxide is described
Q84: For a reaction with a second order
Q85: The rate of disappearance of HBr in
Q86: For the reaction: A → Products concentration-time
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents