The elementary reaction 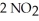 (g) → 2 NO(g) +
(g) → 2 NO(g) + 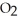 (g) Is second order in
(g) Is second order in 
And the rate constant at 501 K is 7.93 × 10-3 
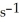 .The reaction half-life at this temperature when
.The reaction half-life at this temperature when 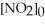 = 0.45 mol L-1 is ________ s.
= 0.45 mol L-1 is ________ s.
A) 3.6 × 10-3
B) 0.011
C) 126
D) 87
E) 280
Correct Answer:
Verified
Q108: Q109: The isomerization of methylisonitrile to acetonitrile Q110: The decomposition of Q111: The reaction that occurs in a Breathalyzer,a Q112: The second-order reaction 2 Mn(CO)5 → Mn2(CO)10 Q114: For the first-order reaction,2 N2O(g)→ 2 N2(g)+ Q115: In aqueous solution,hypobromite ion,BrO-,reacts to produce bromate Q116: The rate constant,k,for a first-order reaction is Q117: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has Q118: The isomerization of methylisonitrile to acetonitrile ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents