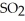
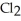 Decomposes in the gas phase by the reaction
Decomposes in the gas phase by the reaction 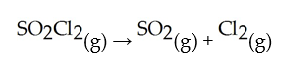
The reaction is first order in 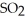
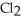 And the rate constant is 3.0 ×
And the rate constant is 3.0 × 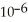
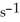 At 600 K.A vessel is charged with 2.4 atm of
At 600 K.A vessel is charged with 2.4 atm of 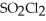 At 600 K.The partial pressure of
At 600 K.The partial pressure of 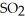
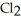 At
At 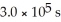 Is ________ atm.
Is ________ atm.
A) 0.76
B) 2.2
C) 0.98
D) 0.29
E) 1.4 × 105
Correct Answer:
Verified
Q103: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q104: Carbon-14,which is present in all living tissue,radioactively
Q105: Nitrogen dioxide decomposes at 300 °C via
Q106: The isomerization reaction,CH3NC → CH3CN,is first order
Q107: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q109: The isomerization of methylisonitrile to acetonitrile
Q110: The decomposition of Q111: The reaction that occurs in a Breathalyzer,a Q112: The second-order reaction 2 Mn(CO)5 → Mn2(CO)10 Q113: The elementary reaction ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents