On forming the nuclide from the protons,neutrons and electrons,the mass loss per nucleon for the 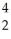 He is 0.00759 amu,whereas that for
He is 0.00759 amu,whereas that for 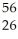 Fe is 0.00944 amu.This means that:
Fe is 0.00944 amu.This means that:
A) 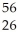 Fe undergoes nuclear fission
Fe undergoes nuclear fission
B) 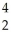 He is more stable than
He is more stable than 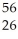 Fe
Fe
C) thirteen 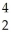 He nuclei could react to form one
He nuclei could react to form one 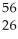 Fe nucleus
Fe nucleus
D) 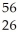 Fe is more stable than
Fe is more stable than 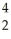 He
He
E) 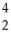 He weighs more than
He weighs more than 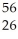 Fe
Fe
Correct Answer:
Verified
Q40: The masses of Q41: Nuclear binding energy is: Q42: Which of the following nuclides is most Q43: An isotope which has too high a Q44: Find the INCORRECT nuclear reaction. Q46: Which of the following is true concerning Q47: Choose the correct statement. Q48: Choose the INCORRECT statement. Q49: In the reaction Q50: Choose the correct word to describe the![]()
I.the energy liberated when
A) 
A)Instability leading to decay
A)Fission is the combining![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents