In the reaction 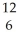 C +
C + 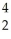 He →
He → 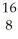 O
O
how much energy is produced per gram of carbon used?
Masses,in unified atomic mass units are: 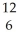 C= 12.00000,
C= 12.00000, 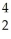 He= 4.00260,
He= 4.00260, 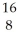 O = 15.99491.
O = 15.99491.
Express your answer in Joules/gram of carbon.
A) 2.10 × 1019 J/g
B) 5.76 × 1010 J/g
C) 7.77 × 104 J/g
D) 2.80 × 1011 J/g
E) 3.36 × 1015 J/g
Correct Answer:
Verified
Q44: Find the INCORRECT nuclear reaction.
A) 
Q45: On forming the nuclide from the protons,neutrons
Q46: Which of the following is true concerning
Q47: Choose the correct statement.
A)Instability leading to decay
Q48: Choose the INCORRECT statement.
A)Fission is the combining
Q50: Choose the correct word to describe the
Q51: Choose the INCORRECT statement.
A)The nuclear binding energy
Q52: Choose the correct word to describe the
Q53: The nuclear binding energy for cobalt-59 is
Q54: What total energy is released when 0.75
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents