The bond enthalpy of the Br-Cl bond is equal to H° for the reaction
BrCl(g) Br(g) + Cl(g) .
Use the following data to find the bond enthalpy of the Br-Cl bond. 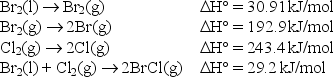
A) 14.6 kJ/mol
B) 203.5 kJ/mol
C) 219.0 kJ/mol
D) 438.0 kJ/mol
E) 407.0 kJ/mol
Correct Answer:
Verified
Q81: The heat of solution of ammonium chloride
Q82: The residential rate for natural gas is
Q83: The heat of solution of NH4NO3 is
Q84: The heat released when one mole of
Q85: The heat of solution of calcium chloride
Q87: Ozone (O3)in the atmosphere can be
Q88: Aluminum oxide can be reduced to
Q89: The heat of combustion of propane, C3H8,
Q90: Given the following
Q91: The heat of solution of ammonium nitrate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents