Given the following H° values,
H2(g)+ 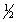 O2(g) H2O(l), H°f = -285.8 kJ/mol
O2(g) H2O(l), H°f = -285.8 kJ/mol
H2O2(l), H2(g)+ O2(g), H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 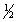 O2(g),
O2(g),
Correct Answer:
Verified
Q85: The heat of solution of calcium chloride
Q86: The bond enthalpy of the Br-Cl
Q87: Ozone (O3)in the atmosphere can be
Q88: Aluminum oxide can be reduced to
Q89: The heat of combustion of propane, C3H8,
Q91: The heat of solution of ammonium nitrate
Q92: The enthalpy of combustion of acetylene
Q93: A 0.3423 g sample of pentane, C5H12,
Q94: A 26.2 g piece of copper metal
Q95: Define specific heat.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents