Calculate the Energy Change for the Reaction K(g)+ I(g) K+(g)+ I - (G)
Given the Following Ionization Energy (IE)and
Calculate the energy change for the reaction K(g) + I(g) K+(g) + I - (g)
Given the following ionization energy (IE) and electron affinity (EA) values. 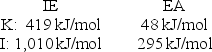
A) -124 kJ/mol
B) -715 kJ/mol
C) 715 kJ/mol
D) 1429 kJ/mol
E) None of these
Correct Answer:
Verified
Q28: Which of the elements listed below would
Q30: What type of chemical bond holds the
Q32: A nonpolar covalent bond (i.e., pure covalent)
Q34: Use the Born-Haber cycle to calculate
Q35: Define electronegativity:
A) an atoms ability to attract
Q36: Arrange the elements F, P, and Cl
Q37: Arrange the elements C, O, and H
Q37: Arrange the following bonds in order of
Q38: Which of the elements listed below would
Q40: Which of the following covalent bonds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents