Use the Born-Haber cycle to calculate the lattice energy of MgO (s) given the following data: H(sublimation) Mg = 130 kJ/mol
I1 (Mg) = 738.1 kJ/mol
I2 (Mg) = 1450 kJ/mol
Bond energy (O=O) = 498.7 kJ/mol
EA (O) = 141 kJ/mol
EA (O-) = -780 kJ/mol
H 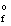
(MgO(s) ) = -601.8 kJ/mol
A) 2200 kJ/mol
B) 2800 kJ/mol
C) 3200 kJ/mol
D) 3800 kJ/mol
E) 4100 kJ/mol
Correct Answer:
Verified
Q21: Which of the elements listed below would
Q28: Which of the elements listed below would
Q32: Calculate the energy change for the
Q32: A nonpolar covalent bond (i.e., pure covalent)
Q36: Arrange the elements F, P, and Cl
Q37: Arrange the elements C, O, and H
Q37: Arrange the following bonds in order of
Q38: Which of the elements listed below would
Q38: Use the Born-Haber cycle to calculate
Q40: Which of the following covalent bonds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents