Hydrogen iodide decomposes according to the equation 2HI(g) 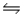 H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of H2 at equilibrium.
H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of H2 at equilibrium.
A) 0.275 M
B) 0.138 M
C) 0.0275 M
D) 0.0550 M
E) 0.220 M
Correct Answer:
Verified
Q23: Kp for the reaction of SO2(g)with O2
Q24: Kp for the reaction CO2(g)+ C(s)
Q25: For the nitrogen fixation reaction 3H2(g)+ N2(g)
Q26: At 400ºC, Kc = 64 for the
Q27: At 340 K, Kp = 69 for
Q29: Kp for the reaction 4CuO(s) 
Q30: At 35ºC, the equilibrium constant for the
Q31: At 700 K, the reaction 2SO2(g)+ O2(g)
Q32: Phosgene, COCl2, a poisonous gas, decomposes according
Q33: Equilibrium is established for the reaction 2X(s)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents