In which of these gas-phase equilibria is the yield of products increased by increasing the total pressure on the reaction mixture?
A) CO(g) + H2O(g) 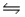 CO2(g) + H2(g)
CO2(g) + H2(g)
B) 2NO(g) + Cl2(g) 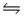 2NOCl(g)
2NOCl(g)
C) 2SO3(g) 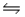 2SO2(g) + O2(g)
2SO2(g) + O2(g)
D) PCl5(g) 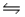 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
E) H2(g) + I2(g) 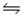 2HI(g)
2HI(g)
Correct Answer:
Verified
Q47: For the reaction H2(g)+ I2(g) 
Q48: For the following reaction at equilibrium,
Q49: For the reaction at equilibrium: 3Fe(s)+ 4H2O(g)
Q50: For the reaction at equilibrium 2SO3
Q51: Consider this gas phase equilibrium system:
Q53: For the reaction PCl3(g)+ Cl2(g) 
Q54: Which of these situations will result if
Q55: For the following reaction at equilibrium
Q56: For the reaction at equilibrium: CO(g)+ H2O(g)
Q57: Concerning the following reaction at equilibrium: 3Fe(s)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents