For the reaction at equilibrium: CO(g) + H2O(g) 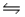 CO2(g) + H2(g) , increasing the volume of the container would:
CO2(g) + H2(g) , increasing the volume of the container would:
A) Shift the equilibrium to the right
B) Shift the equilibrium to the left
C) Increase the value of the equilibrium constant, K
D) Decrease the value of the equilibrium constant, K
E) No change
Correct Answer:
Verified
Q51: Consider this gas phase equilibrium system:
Q52: In which of these gas-phase equilibria is
Q53: For the reaction PCl3(g)+ Cl2(g) 
Q54: Which of these situations will result if
Q55: For the following reaction at equilibrium
Q57: Concerning the following reaction at equilibrium: 3Fe(s)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents