For the reaction 2NOCl(g) 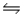 2NO(g) + Cl2(g) , Kc = 8.0 at a certain temperature.What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?
2NO(g) + Cl2(g) , Kc = 8.0 at a certain temperature.What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?
A) 1.26 M
B) 2.25 M
C) 2.50 M
D) 3.52 M
E) 11.0 M
Correct Answer:
Verified
Q53: For the reaction PCl3(g)+ Cl2(g) 
Q54: Which of these situations will result if
Q55: For the following reaction at equilibrium
Q56: For the reaction at equilibrium: CO(g)+ H2O(g)
Q57: Concerning the following reaction at equilibrium: 3Fe(s)+
Q59: For the equilibrium reaction 2SO2(g)+ O2(g)
Q60: Consider this reaction at equilibrium: 2SO2(g)+
Q61: Which of the following rate laws
Q62: Solid ammonium hydrogen sulfide is introduced into
Q63: The equilibrium constants for the chemical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents