Multiple Choice
Consider this reaction at equilibrium: 2SO2(g) + O2(g) 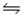
2SO3(g) , Hºrxn = -198 kJ/mol
If the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
A) A shift to produce more SO2
B) A shift to produce more O2
C) No change
D) A shift to produce more SO3
Correct Answer:
Verified
Related Questions
Q55: For the following reaction at equilibrium
Q56: For the reaction at equilibrium: CO(g)+ H2O(g)
Q57: Concerning the following reaction at equilibrium: 3Fe(s)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents