If the reaction 2H2S(g) 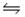
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.0120.Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g) ?
A) 1.06 atm
B) 1.36 atm
C) 2.39 atm
D) 4.20 atm
E) 1.51 atm
Correct Answer:
Verified
Q60: Consider this reaction at equilibrium: 2SO2(g)+
Q61: Which of the following rate laws
Q62: Solid ammonium hydrogen sulfide is introduced into
Q63: The equilibrium constants for the chemical
Q64: A solution was prepared such that the
Q66: Consider the reaction, N2(g)+ 3H2(g) 
Q67: Consider the reaction N2(g)+ 3H2(g) 
Q68: Given the following data for the reaction:
Q69: Consider the reaction N2(g)+ 3H2(g) 
Q70: Consider the reaction N2(g)+ 3H2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents