Consider the reaction, N2(g)+ 3H2(g) 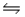
2NH3(g).Kc = 8.1 x 10-3 at 900 K.
What is the value of Kc for NH3(g) 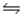
½N2(g)+ 3/2H2(g)
Correct Answer:
Verified
Q61: Which of the following rate laws
Q62: Solid ammonium hydrogen sulfide is introduced into
Q63: The equilibrium constants for the chemical
Q64: A solution was prepared such that the
Q65: If the reaction 2H2S(g)
Q67: Consider the reaction N2(g)+ 3H2(g) 
Q68: Given the following data for the reaction:
Q69: Consider the reaction N2(g)+ 3H2(g) 
Q70: Consider the reaction N2(g)+ 3H2(g) 
Q71: Consider the reaction N2(g)+ 3H2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents