Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) 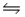
2SO3(g)
Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished.The new equilibrium total pressure will be
A) twice P1
B) three times P1
C) 3.5 P1
D) less than twice P1
E) unchanged
Correct Answer:
Verified
Q37: At 700 K, the reaction 2SO2(g)+ O2(g)
Q38: Sodium carbonate, Na2CO3(s), can be prepared by
Q39: Hydrogen iodide decomposes according to the equation
Q40: Consider the following equilibria: 2SO3(g) 
Q41: For the following reaction at equilibrium,
Q43: For the following reaction at equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents