Given the following Ka values, which anion is the strongest base? 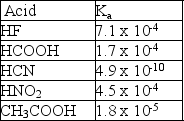
A) F-
B) HCOO-
C) CN-
D) NO2-
E) CH3COO-
Correct Answer:
Verified
Q61: Which one of these net ionic
Q64: Arrange the acids HOCl, HClO3, and HClO2
Q67: Which one of the following equations represents
Q68: Arrange the acids HOBr, HBrO3, and HBrO2
Q69: Which one of the following equations represents
Q71: Calculate the pOH for a solution with
Q75: Which solution will have the lowest pH
A)
Q77: Which one of the following statements is
Q78: Which one of these net ionic
Q80: Which solution will have the lowest pH
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents