Which one of the following equations represents the ionization of a weak base in water?
A) NH3(aq) + H2O(l) 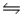 NH2+(aq) + H3O+(aq)
NH2+(aq) + H3O+(aq)
B) NH3(aq) + H2O(l) 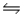 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
C) NH3(aq) + H2O(l) 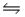 NH4- (aq) + OH+(aq)
NH4- (aq) + OH+(aq)
D) NH3(aq) + OH-(aq) 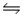 NH2- (aq) + H2O(l)
NH2- (aq) + H2O(l)
E) NH3(aq) + H3O+(aq) 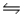 NH4+(aq) + H2O(l)
NH4+(aq) + H2O(l)
Correct Answer:
Verified
Q61: Which one of these net ionic
Q62: The equilibrium expression for the ionization of
Q62: Given the following Ka values, which anion
Q63: Given the following Kb values, which cation
Q65: Which one of these equations represents
Q68: Arrange the acids HOBr, HBrO3, and HBrO2
Q69: Which one of the following equations represents
Q72: Given the following Ka values, which anion
Q73: When comparing acid strength of binary acids
Q80: Which solution will have the lowest pH
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents