In the reaction CaO(s) + SO2(g) 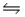 CaSO3(s) ,
CaSO3(s) ,
A) O2- acts as a Lewis base, and SO2 acts as a Lewis acid.
B) Ca2+ acts as a Lewis base, and SO42- acts as a Lewis acid.
C) SO42- acts as a Lewis base, and SO2 acts as a Lewis acid.
D) SO2 acts as a Lewis base, and O2- acts as a Lewis acid.
E) SO2 acts as a Lewis base, and Ca2+ acts as a Lewis acid.
Correct Answer:
Verified
Q79: Arrange the acids H2Se, H2Te, and H2S
Q80: Arrange the acids HBr, H2Se, and H3As
Q82: Predict the direction in which the equilibrium
Q83: For H3PO4, Ka1 = 7.3 × 10-3,
Q85: The oxides CO2 and SO3 will form
Q86: Calculate the concentration of chromate ion (CrO42-)in
Q88: Which of the following is both a
Q88: Calculate the concentration of oxalate ion (C2O42-)in
Q89: Which of the following yields a basic
Q100: Which of the following is both a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents