The salt hydrolysis reaction for NH4NO3 that represents the acidic or basic nature of the solution is:
A) NH4+(aq) + H2O(l) 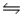 NH3(aq) + H2(g) + OH-(aq)
NH3(aq) + H2(g) + OH-(aq)
B) NO3-(aq) + H2O(l) 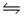 HNO3(aq) + OH-(aq)
HNO3(aq) + OH-(aq)
C) NH4+(aq) + H2O(l) 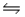 H3O+(aq) + NH3(aq)
H3O+(aq) + NH3(aq)
D) NH4+(aq) + NO3-(aq) 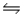 NH4NO3(aq)
NH4NO3(aq)
E) NO3-(aq) + 3H2O(l) 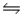 HNO3(aq) + 2H3O+(aq)
HNO3(aq) + 2H3O+(aq)
Correct Answer:
Verified
Q83: The equilibrium expression for the hydrolysis of
Q89: Which of these species will act as
Q95: The oxides SO2 and N2O5 will form
Q100: Predict the direction in which the equilibrium
Q101: The pH of a 0.14 M solution
Q102: Calculate the pH of a 0.18 M
Q106: Which one of these salts will form
Q106: The salt hydrolysis reaction for CH3COONa that
Q113: What is the pH of a 0.30
Q114: Which one of these salts will form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents