The salt hydrolysis reaction for CH3COONa that represents the acidic or basic nature of the solution is:
A) Na+(aq) + H2O(l) 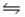 NaH(s) + OH-(aq)
NaH(s) + OH-(aq)
B) CH3COO-(aq) + H2O(l) 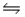 CH2COO2-(aq) + H3O+(aq)
CH2COO2-(aq) + H3O+(aq)
C) CH3COO-(aq) + H2O(l) 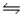 CH3COOH(aq) + OH-(aq)
CH3COOH(aq) + OH-(aq)
D) Na2O(s) + H2O(l) 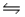 2NaOH(aq)
2NaOH(aq)
E) CH3COO-(aq) + Na+(aq) 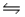 CH3COONa
CH3COONa
Correct Answer:
Verified
Q101: The pH of a 0.14 M solution
Q102: Calculate the pH of a 0.18 M
Q102: The salt hydrolysis reaction for NH4NO3 that
Q106: Which one of these salts will form
Q108: Calculate the pH of a 0.055 M
Q109: Calculate the pH of a 0.021 M
Q110: For H3PO4, Ka1 = 7.3 × 10-3,
Q113: What is the pH of a 0.30
Q114: Which one of these salts will form
Q116: Calculate the pH of a 0.20 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents