List the number of protons, neutrons, and nucleons (protons + neutrons) , in that order, for an isotope with the symbol: 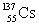
A) 137, 55, 192
B) 55, 137, 192
C) 55, 82, 137
D) 82, 55, 137
E) 82, 137, 219
Correct Answer:
Verified
Q1: Consider the following decay series:
Q2: Consider the following decay series:
Q2: Alpha particles are identical to
A) protons.
B) helium
Q3: List the number of protons, neutrons, and
Q5: Which of the following is characteristic of
Q6: Consider the following decay series: 
Q8: Consider the following decay series: 
Q10: As a result of beta decay, the
Q10: When atoms of aluminum-27 are bombarded with
Q11: Consider the following decay series:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents