List the number of protons, neutrons, and nucleons (protons + neutrons) , in that order, for an isotope with the symbol: 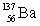
A) 56, 81, 137
B) 81, 56, 137
C) 56, 137, 193
D) 137, 56, 193
E) 81, 137, 218
Correct Answer:
Verified
Q1: Consider the following decay series:
Q2: Consider the following decay series:
Q2: Alpha particles are identical to
A) protons.
B) helium
Q4: List the number of protons, neutrons, and
Q5: Which of the following is characteristic of
Q6: Consider the following decay series: 
Q8: Consider the following decay series: 
Q10: As a result of beta decay, the
Q10: When atoms of aluminum-27 are bombarded with
Q11: Consider the following decay series:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents