How much energy (heat) is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 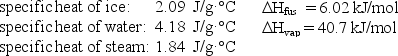
A) 2,570 kJ
B) 1,086 kJ
C) 157.8 kJ
D) 40.2 kJ
E) 22,957 kJ
Correct Answer:
Verified
Q49: Potassium crystallizes in a body-centered cubic lattice.
Q57: Use the graph of vapor pressure to
Q58: Silver metal crystallizes in a face-centered cubic
Q61: What mass of water would need
Q62: Acetic acid has a heat of fusion
Q63: The normal boiling point of methanol (CH3OH)is
Q64: The vapor pressure of ethanol is 400
Q65: Which one of the following elements would
Q67: The molar enthalpy of vaporization of carbon
Q81: Octane is a liquid component of gasoline.Given
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents