For the nitrogen fixation reaction 3H2(g) + N2(g) 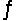 2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
A) 0.750 M
B) 2.7 M
C) 0.250 M
D) 0.025 M
E) 1.85 M
Correct Answer:
Verified
Q26: At 400ºC, Kc = 64 for the
Q27: For the following reaction at equilibrium,
Q28: For the reaction SO2(g)+ NO2(g) 
Q29: Consider the reaction N2(g)+ O2(g) 
Q30: For the following reaction at equilibrium
Q32: Consider the following reactions and their associated
Q33: For the reaction H2(g)+ I2(g) 
Q34: At 35ºC, the equilibrium constant for the
Q35: Hydrogen iodide decomposes according to the equation
Q36: At 700 K, the reaction 2SO2(g)+ O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents