Consider the following reactions and their associated equilibrium constants: 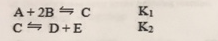
For the reaction A + 2B  D + E, having equilibrium constant Kc,
D + E, having equilibrium constant Kc,
A) Kc = K1 + K2
B) Kc = K1/K2
C) Kc = K1 - K2
D) Kc = (K1) (K2)
E) Kc = K2/K1
Correct Answer:
Verified
Q27: For the following reaction at equilibrium,
Q28: For the reaction SO2(g)+ NO2(g) 
Q29: Consider the reaction N2(g)+ O2(g) 
Q30: For the following reaction at equilibrium
Q31: For the nitrogen fixation reaction 3H2(g)+ N2(g)
Q33: For the reaction H2(g)+ I2(g) 
Q34: At 35ºC, the equilibrium constant for the
Q35: Hydrogen iodide decomposes according to the equation
Q36: At 700 K, the reaction 2SO2(g)+ O2(g)
Q37: At 700 K, the reaction 2SO2(g)+ O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents