When the substances in the equation below are at equilibrium, at pressure P and temperature T, the equilibrium can be shifted to favor the products by CuO(s) + H2(g) 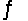
H2O(g) + Cu(s) Hºrxn = -2.0 kJ/mol
A) increasing the pressure by means of a moving piston at constant T.
B) increasing the pressure by adding an inert gas such as nitrogen.
C) decreasing the temperature.
D) allowing some gases to escape at constant P and T.
E) adding a catalyst.
Correct Answer:
Verified
Q53: Consider this gas phase equilibrium system:
Q54: When the reaction 2H2S(g) Q55: A solution was prepared such that the Q56: Describe why addition of a catalyst does Q57: Solid ammonium hydrogen sulfide is introduced into![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
