Multiple Choice
Consider this reaction at equilibrium: 2SO2(g) + O2(g) 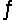
2SO3(g) , Hºrxn = -198 kJ/mol
If the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
A) A shift to produce more SO2
B) A shift to produce more O2
C) No change
D) A shift to produce more SO3
Correct Answer:
Verified
Related Questions
Q55: A solution was prepared such that the
Q56: Describe why addition of a catalyst does
Q57: Solid ammonium hydrogen sulfide is introduced into
Q58: When the substances in the equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
