Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J 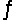
CO(g)+ H2(g).What will happen to the mass of carbon if we add gaseous water to the system?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q59: The reaction 2NO(g) Q60: Consider this reaction at equilibrium: 2SO2(g)+ Q61: Consider the equilibrium equation C(s)+ H2O(g)+ 2296 Q62: Consider the chemical reaction 2NH3(g) Q63: Ethanol and acetic acid react to form Q65: Consider the reaction N2(g)+ 3H2(g) Q66: The data below refer to the following Q67: Consider the chemical reaction 2NH3(g) Q68: Consider the reaction N2(g)+ 3H2(g) Q85: What conditions are used in the Haber![]()




Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents