Consider the chemical reaction 2NH3(g) 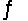
N2(g)+ 3H2(g).The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present.Estimate the equilibrium concentration of N2(g).
Correct Answer:
Verified
Q57: Solid ammonium hydrogen sulfide is introduced into
Q58: When the substances in the equation
Q59: The reaction 2NO(g) Q60: Consider this reaction at equilibrium: 2SO2(g)+ Q61: Consider the equilibrium equation C(s)+ H2O(g)+ 2296 Q63: Ethanol and acetic acid react to form Q64: Consider the equilibrium equation C(s)+ H2O(g)+ 2296 Q65: Consider the reaction N2(g)+ 3H2(g) Q66: The data below refer to the following Q67: Consider the chemical reaction 2NH3(g) ![]()


Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents