Consider the following equilibrium,
4NH3(g)+ 3O2(g) 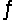
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the products would increase, decrease, or remain constant when the temperature is increased.
Correct Answer:
Verified
Q90: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q91: What is the correct equilibrium constant expression
Q92: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q93: Two moles of PCl5 are placed in
Q94: The equilibrium constant for the chemical equation
2NO(g)+
Q95: The equilibrium constant expression for the reaction
CuO(s)+
Q96: If the system 3H2(g)+ N2(g) 
Q97: When the reaction 2O3(g) Q98: For the reaction H2(g)+ I2(g) Q100: Equilibrium constants are known for the following![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents