Equilibrium constants are known for the following reactions:
S(s)+ 3/2O2(g) 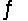
SO3(g)Kc = 9.2 * 1023
SO3(g) 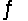
SO2(g)+ 1/2O2(g)Kc = 4.8 *10- 4
Thus, for the reaction S(s)+ O2(g) 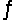
SO2(g), Kc = 4.4 * 1020.
Correct Answer:
Verified
Q90: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q91: What is the correct equilibrium constant expression
Q92: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q93: Two moles of PCl5 are placed in
Q94: The equilibrium constant for the chemical equation
2NO(g)+
Q95: The equilibrium constant expression for the reaction
CuO(s)+
Q96: If the system 3H2(g)+ N2(g) 
Q97: When the reaction 2O3(g) Q98: For the reaction H2(g)+ I2(g) Q99: Consider the following equilibrium,![]()

4NH3(g)+ 3O2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents