Nitrosyl chloride (NOCl) decomposes at elevated temperatures according to the equation 2NOCl(g) 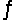 2NO(g) + Cl2(g) . Calculate Kp for this reaction at 227°C.( H° = 81.2 kJ/mol, S° = 128 J/K·mol)
2NO(g) + Cl2(g) . Calculate Kp for this reaction at 227°C.( H° = 81.2 kJ/mol, S° = 128 J/K·mol)
A) 1.59 10-2
B) 2.10 10-7
C) 62.8
D) 4.90 106
E) 3.20 109
Correct Answer:
Verified
Q25: The normal freezing point of ammonia
Q25: Sodium carbonate can be made by
Q26: Determine the equilibrium constant Kp at
Q27: Determine the equilibrium constant (Kp)at 25°C
Q29: The element oxygen was prepared by
Q30: At 1500°C the equilibrium constant for
Q31: The equilibrium constant at 427°C for
Q33: A negative sign for
Q34: Calculate the equilibrium constant for the
Q40: For the reaction H2(g)+ S(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents