For the reaction CuS(s)+ H2(g) 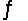 H2S(g)+ Cu(s),
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: Hydrogen peroxide (H2O2)decomposes according to the
Q37: The equilibrium constant for the reaction
Q38: Ozone (O3)in the atmosphere can reaction
Q39: A spontaneous endothermic reaction always
A)causes the surroundings
Q40: Kw for the auto-ionization of water,
Q43: Using the thermodynamic data provided below, calculate
Q44: Find the temperature at which Kp
Q45: In the gas phase, formic acid
Q46: Which species will have the greatest absolute
Q53: The reaction rates of many spontaneous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents