Short Answer
For the reaction 3H2(g)+ N2(g) 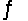 2NH3(g), Kc = 9.0 at 350°C. What is the value of G at this temperature when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
2NH3(g), Kc = 9.0 at 350°C. What is the value of G at this temperature when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
Correct Answer:
Verified
Related Questions
Q64: Predict the sign of
Q67: For the reaction SbCl5(g) 
Q68: For the reaction SbCl5(g) 
Q71: Assuming
Q72: The free energy of formation of nitric
Q73: For the reaction 3H2(g)+ N2(g) 
Q74: Predict the sign of
Q85: Calculate the free energy of formation
Q99: For a certain reaction,
Q100: The heat of vaporization of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents