For the reaction SbCl5(g) 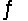 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate G at 800 K and 1 atm pressure (assume S° and H° do not change with temperature).
Correct Answer:
Verified
Q63: For the reaction SbCl5(g) 
Q64: Predict the sign of
Q68: For the reaction SbCl5(g) 
Q69: For the reaction 3H2(g)+ N2(g)
Q71: Assuming
Q72: The free energy of formation of nitric
Q82: Is the reaction SiO2(s)+ Pb(s)
Q85: Calculate the free energy of formation
Q99: For a certain reaction,
Q100: The heat of vaporization of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents