For the reaction SbCl5(g) 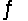 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Correct Answer:
Verified
Q58: For the reaction CuS(s)+ H2(g)
Q59: Find the temperature at which the reaction
Q60: The standard free energy of formation of
Q64: Predict the sign of
Q67: For the reaction SbCl5(g) 
Q68: For the reaction SbCl5(g) 
Q71: Assuming
Q82: Is the reaction SiO2(s)+ Pb(s)
Q86: What is the free energy change
Q100: The heat of vaporization of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents