In two dimensions sulfuric acid,  S
S  , is often written as shown below. What three-dimensional shape does this molecule most likely have?
, is often written as shown below. What three-dimensional shape does this molecule most likely have? 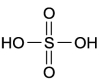
A) tetrahedral
B) octahedral
C) square pyramidal
D) square planar
Correct Answer:
Verified
Q83: Which bond is most polar?
A)H-N
B)N-C
C)C-C
D)O-H
Q115: How many substituents are on the following
Q116: Which of the following molecules has two
Q117: What is the molecular shape of the
Q118: Phosphine is a covalent compound that contains
Q119: How many electrons are used to draw
Q121: Which covalent bond is more polar: a
Q122: Two molecules, A and B, have very
Q123: Which of the following statements best describes
Q125: Consider the electron-dot geometries of P
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents