An individual carbon-oxygen bond is polar. Yet carbon dioxide, C 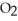 , which has two carbon-oxygen bonds, is nonpolar because ________.
, which has two carbon-oxygen bonds, is nonpolar because ________.
A) the molecule has an even number of electrons
B) it has a greater symmetry
C) the electron-pulls of the two oxygen atoms are equal and opposite
D) Two of the above are reasonable
Correct Answer:
Verified
Q101: List the following bonds in order of
Q105: Which molecule is most polar?
A)S=C=S
B)O=C=O
C)O=C=S
D)These all have
Q120: Which of the following statements describes a
Q144: Which of the following molecules is the
Q145: Which of the following molecules should have
Q146: Three kids sitting equally apart around a
Q147: Would ammonia, N Q149: A substance consisting of which molecule shown Q151: Which shows atoms in order of increasing Q152: Water, ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents