How many moles of water, 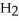 O, are produced from the reaction of 16 grams methane, C
O, are produced from the reaction of 16 grams methane, C 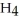 , with an unlimited supply of oxygen,
, with an unlimited supply of oxygen, 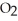 . How many grams of
. How many grams of 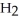 O is this? C
O is this? C 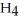 + 2
+ 2 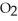 → C
→ C 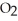 + 2
+ 2 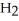 O
O
A) 0.889 mole, which is 16 grams
B) 2.0 moles of water, which is 32 grams
C) 2.0 moles of water, which is 36 grams
D) 1.0 mole of water, which is 18 grams
Correct Answer:
Verified
Q90: Use the bond energies below to determine
Q98: How much energy, in kilojoules, is released
Q98: What is an endothermic reaction?
A)It is a
Q101: Which is higher in an endothermic reaction:
Q103: Given that the bond energy of N2
Q105: How many bonds between nitrogen and hydrogen
Q109: If it takes energy to break bonds
Q113: Which of the following reaction energies is
Q114: Which of the following reaction energies is
Q120: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents