How much energy, in kilojoules, is released or absorbed from the reaction of one mole of nitrogen, 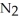 , with three moles of molecular hydrogen,
, with three moles of molecular hydrogen, 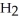 , to form two moles of ammonia, N
, to form two moles of ammonia, N 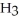 ? H-N (bond energy: 389 kJ/mol)
? H-N (bond energy: 389 kJ/mol)
H-H (bond energy: 436 kJ/mol)
N  N (bond energy: 946 kJ/mol)
N (bond energy: 946 kJ/mol)
N  N + H-H + H-H → N
N + H-H + H-H → N 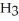 + N
+ N 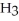
A) +899 kJ/mol absorbed
B) -993 kJ/mol released
C) +80 kJ/mol absorbed
D) -80 kj/mol released
Correct Answer:
Verified
Q95: How many moles of water, 
Q98: What is an endothermic reaction?
A)It is a
Q101: Which is higher in an endothermic reaction:
Q101: Which of the above reactions would proceed
Q102: Which of the following statements is not
Q103: Given that the bond energy of N2
Q103: For the above energy profiles, which reaction
Q113: Which of the following reaction energies is
Q114: Which of the following reaction energies is
Q120: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents