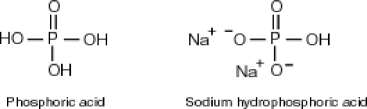
Why is phosphoric acid, 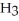
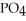 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 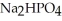 ?
? 
A) Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B) Some of the released sodium ions in 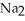 HP
HP 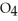 form NaOH (a base) , which decreases the acidity of the
form NaOH (a base) , which decreases the acidity of the 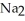 HP
HP 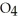 .
.
C) Phosphoric acid dissociates 100% in water whereas 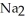 HP
HP  only dissociates about 50%.
only dissociates about 50%.
D) None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.
Correct Answer:
Verified
Q30: What is the main characteristic of a
Q40: What is the main characteristic of a
Q53: Which of the following statements about strong
Q56: Which should be a stronger base: ammonia,
Q57: A weak acid is added to a
Q58: Which of the above images would best
Q59: Arrange the following images of an aqueous
Q60: Does a water molecule become more or
Q61: What would the concentration of OH- be
Q62: Which is the stronger acid: H-F or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents