Which is the stronger acid: H-F or H-I?
A) H-F, because the electronegativity of fluorine is higher than that of iodine making the H-F bond more polar and more likely to release the hydrogen ion to solution.
B) H-F, because acid strength for binary acids decreases "down the group" of the periodic table.
C) H-I, because iodine releases the hydrogen in order to form 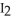 (s) at room temperature.
(s) at room temperature.
D) H-I, because the H-I bond is weaker and so it is easier to break to form hydrogen ions.
Correct Answer:
Verified
Q48: Q57: Why is phosphoric acid, Q58: Which of the above images would best Q59: Arrange the following images of an aqueous Q60: Does a water molecule become more or Q61: What would the concentration of OH- be Q63: In behaving as both an acid and Q65: Qualitatively, what happens to the hydroxide ion Q66: Qualitatively, what happens to the hydronium ion Q67: What is pH?![]()
![]()
A)It is a numerical scale
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents