The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 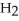 O +
O + 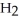 O →
O → 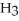
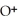 + O
+ O 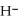 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 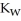 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
A) The value of 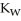 will stay the same, since it is a constant.
will stay the same, since it is a constant.
B) The value of 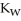 will decrease, since there are more hydronium ions than hydroxide ions.
will decrease, since there are more hydronium ions than hydroxide ions.
C) The value of 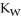 will increase since the rise in temperature allows for a higher concentration of both ions.
will increase since the rise in temperature allows for a higher concentration of both ions.
D) The value of 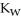 will decrease since there are more hydroxide ions than hydronium ions.
will decrease since there are more hydroxide ions than hydronium ions.
Correct Answer:
Verified
Q55: Which of the following solutions is the
Q72: What happens to the pH of an
Q92: Pour vinegar onto beach sand from the
Q94: Along with the pH scale, there is
Q95: What would be the concentration of hydronium
Q96: If you had a 1 M solution
Q100: When the hydronium ion concentration equals 10
Q101: Carbon dioxide reacts with water to form
Q102: When a hydronium ion concentration equals 1
Q103: When the pH of a solution is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents