What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
A) Concentration of 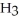
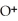 = 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
= 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
B) Concentration of 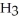
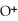 = 30 M. It is impossible because pH can not be negative.
= 30 M. It is impossible because pH can not be negative.
C) Concentration of 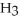
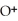 =
= 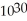 M. It is impossible because any container used to hold the acid would immediately corrode.
M. It is impossible because any container used to hold the acid would immediately corrode.
D) Concentration of 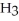
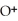 =
= 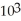 M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
Correct Answer:
Verified
Q55: Which of the following solutions is the
Q63: If you had a 1 M solution
Q72: What happens to the pH of an
Q90: If you had a 1 M solution
Q91: What does the value of 
Q92: Pour vinegar onto beach sand from the
Q94: Along with the pH scale, there is
Q96: If you had a 1 M solution
Q98: The amphoteric reaction between two water molecules
Q100: When the hydronium ion concentration equals 10
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents