Sometimes an individual going through a traumatic experience cannot stop hyperventilating. In such a circumstance, it is recommended that the individual breath into a paper bag or cupped hands as a useful way to avoid an increase in blood pH, which can cause the person to pass out. Explain how this works.
A) Carbon dioxide initially exhaled is reinhaled to help maintain adequate levels of carbonic acid in the blood.
B) It helps to slow their breathing down to prevent excess oxygen from entering the blood.
C) The paper absorbs C 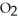 to prevent the accumulation of excess C
to prevent the accumulation of excess C 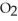 in the blood.
in the blood.
D) The paper bag helps to retain the water vapor leaving the mouth to prevent an increase in the concentration of lactic acid in the body.
Correct Answer:
Verified
Q112: A buffer solution can neutralize _.
A)strong acids
Q113: Hydrogen chloride is added to a buffer
Q114: Which of the following statements about buffers
Q115: What is the hydroxide ion concentration in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents