Hydrogen chloride is added to a buffer solution of ammonia, N 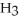 , and ammonium chloride, N
, and ammonium chloride, N 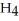 ⁺Cl⁻. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
⁺Cl⁻. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
A) HCl reacts with both the ammonia and ammonium chloride, so the concentration of both decreases.
B) HCl reacts with only the ammonium chloride, so the concentration of the ammonium chloride decreases and the concentration of ammonia increases.
C) HCl reacts with both the ammonia and ammonium chloride, so the concentration of both increases.
D) HCl reacts with only the ammonia, so the concentration of the ammonium chloride increases and the concentration of ammonia decreases.
Correct Answer:
Verified
Q93: What happens to the pH of soda
Q106: In the following buffer system, what happens
Q108: What is the hydroxide ion concentration in
Q109: At what point will a buffer solution
Q112: A buffer solution can neutralize _.
A)strong acids
Q114: Which of the following statements about buffers
Q115: What is the hydroxide ion concentration in
Q116: Sodium bicarbonate, NaHC Q117: Sometimes an individual going through a traumatic Q118: Which of the following items would you![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents